Defective Product Lawsuit
Our law firm helps people who are critically injured by unsafe products. In some of our cases, the product has been recalled, but a recall is not necessary before we file a lawsuit and win a settlement.
A grieving mother hired us when her little one died while in a baby sling. She thought, correctly, that the cause of her baby’s death was the sling, that the design of the sling caused her infant’s face to roll into the fabric, preventing him from breathing.
Attorneys at Pritzker Hageman took the case. Our legal team contacted the federal government about this product and urged the department to force a recall. After only a few weeks, a recall was issued. Our attorneys could have just filed a lawsuit, gotten a settlement and moved on, but we made sure that this product was taken off of the market. This is what we do. We work to get maximum compensation and justice for people injured by defective products and we strive to force needed changes that will protect public safety.
Contact our award-winning defective product legal team for a free consultation with a lawyer.
Phone: 1-888-377-8900 | Text: 612-261-0856
In another case, a medical product was recalled because it could fatally sicken newborns, although the exact cause of the illnesses was not determined. After receiving many calls from parents, our law firm hired experts to find the cause. A renowned professor on the expert team we assembled did an in-depth study of these illnesses and how the product could have caused them. His research was published in a medical journal and our attorneys were able to use that research to win millions in compensation for our injured infant clients and their parents.
While many people might not consider tainted food to be a defective product, it most certainly is. Food is supposed to be safe to eat! In one of our legal cases, a young man consumed food contaminated with a deadly bacteria called Listeria monocytogenes. After a few weeks, the bacteria had traveled to his brain and infected the meninges, the lining of the brain and spinal cord. The sickness this caused is called meningitis, and this young man almost died from it. He contacted our law firm, and our attorneys quickly flew out to meet with him and his family. They found out that, before he got sick, he had eaten a product that had been recalled due to possible Listeria contamination. They pursued a settlement from the company that made the product and won millions in compensation. This money will make a huge difference in being able to deal with the ongoing health consequences that can occur with a Listeria infection.
Our lawyers have won millions for clients injured by defective and dangerous products, including the following 3 recoveries (read about our top cases):
- $45 million for clients who got kidney failure after using an over-the-counter medicine;
- $10 million for babies who contracted a mysterious illness after ingesting a medical product that was later recalled;
- $4.5 million for a client who ate at a restaurant and ended up in the hospital on dialysis due to kidney failure caused by an E. coli infection.
You can ask our product liability lawyers if you have a personal injury or wrongful death claim against a company that sold a defective product.
A Recall Lawsuit Seeks Compensation for Harm from a Defective Product
A recall lawsuit involves personal injury or wrongful death caused by a defective product, one that is not reasonably safe for its intended use. “Intended use” includes all those uses a reasonably prudent person might make, bearing in mind its characteristics, warnings and labels. In many of our recall cases, the company was prompted to act by reports of personal injury or wrongful death. However, on many occasions it has been our attorney’s actions that finally led to a company issuing a recall.
Federal agencies involved may include:
- FDA Recalls: Medical and food;
- USDA-FSIS Recalls: Meat and poultry;
- CPSC Recalls: Consumer.
Most people do not know that the federal government can’t recall products, the agencies above can only urge companies to recall unsafe products. If that does not work, they can take legal steps to force a recall, but it is a slow process.
Just because a product is sold does not mean it is safe.
You may have a lawsuit against the manufacturer of the defective product, the wholesaler or distributor of the product, and often the retailer of the product. These suits involve one or more of the following:
- a bad design that puts consumers at risk of injury or wrongful death;
- a problem with the manufacturing process, either in general or for certain lots (manufacturing runs);
- a label with no warning about this particular risk of injury;
- instructions that do not address the risk and/or how to prevent harm.
Thousands of people receive injuries from recalled items each year. The makers of these unsafe products should be held responsible because that is what is fair. We make sure that happens and work to get settlement money for the following:
- all related medical expenses, even those that are expected in the future;
- income lost because the person has to get a lower paying job or can’t work;
- compensation for pain, emotional suffering, disability, disfigurement, and loss of quality of life.
In situations where company executives and/or managers have behaved very badly, we seek “punitive damages” for our clients, which means that we ask for an additional, large amount of money to punish the company for the intentional or reckless behavior.
What to Do If You or a Loved One Has Been Injured
If you have been injured here are some things to consider:
- Save or retain the suspected item and any documents or packaging that accompanied it.
- Identify as much information about the product as possible including the name of the manufacturer, model name and number, place and date of purchase, and serial number.
- Store it in a safe place and do not modify or change it.
- Obtain the name, address, and telephone number of any witnesses.
- Do not discuss the case with anyone and do not give any written or recorded statements about it.
Product Liability Lawyers
You can call 1-888-377-8900 (toll-free) or click here to contact our lawyers and get your free consultation. Our law firm has a reputation for success in complex lawsuits and is listed in U.S. News and World Report’s The Best Law Firms in America. Our product liability lawyers have been interviewed by The New York Times, The Wall Street Journal, Lawyers USA, and other publications. We have recovered millions of dollars on behalf of people hurt or killed by defective items, including flammable furniture, industrial saws, shredding machines, automobiles, pumps and sprayers, hip implants, and dangerous drugs and other medical, some of which caused severe infections.
We have a national practice and have won money for clients throughout the U.S.
Recalls and Unsafe Products:
Recall for Max Mobility/Permobil Medical Wheelchairs After 3 Serious Injuries from Faulty Speed Control Dial
Max Mobility/Permobil has issued a nationwide recall of the SmartDrive Speed Control Dial used with the SmartDrive MX2+ Power Assist Device. This recall follows 646 complaints and three reports of serious injuries, including fractures of the hip, tibia, and malleolus bone. The Speed Control Dial has been found to cause unintended activation or failure to stop the SmartDrive motor, posing significant risks to users of medical wheelchairs.
The recalled wheelchairs were manufactured and distributed through authorized dealers for purchase by consumers between August 17, 2023, and November 21, 2024. The model numbers include MX2-3DCK/MX2-3DC.
The recall was triggered by a material change in the printed circuit board assembly of the Speed Control Dial, leading to inconsistent performance issues. These issues include the SmartDrive motor continuing to run when it should stop, starting unexpectedly, or stopping unexpectedly. These malfunctions can result in various injuries:
- Serious injuries: Muscle strain or tear, ligament strain or tear, bone fracture, and concussion.
- Serious injuries to others: Bone fractures to individuals standing or sitting near the user, caused by unintentional activation and/or inability to turn off power assist.
- Minor injuries: Skin irritation, minor cuts, and bruises.

Stanley Switchback and Trigger Action Travel Mugs Recalled for Burn Hazard
In December 2024, Stanley issued a travel mug recall for all Stanley Switchback and Trigger Action stainless steel travel mugs sold in the United States for risk of burn injury. The threads on the lid can shrink when exposed to heat and torque, causing the lid to detach during use. Two burn injuries have been reported.
From June 2016 through December 2024, the recalled mugs were sold at retail stores nationwide including Amazon.com, Walmart, Dick’s Sporting Goods, and Target for $20 to $50.
The recall includes double-walled mugs in a variety of colors including white, black, and green, in 12 oz., 16 oz., and 20 oz. sizes with a polypropylene lid.
Switchback
- 12 oz, 20-01437
- 16 oz, product code 20-01436, 20-02211
Trigger Action
- 12 oz, 20-02033, 20-02779, 20-02825z
- 16 oz, 20-02030, 20-02745, 20-02957
- 20 oz, 20-02034, 20-02746
Good Earth Lighting Recalls Over 1 Million Rechargeable Lights Due to Fire Hazard from Lithium-Ion Batteries
In June 2024, Good Earth Lighting issued a recall for more than 1.2 million rechargeable lights due to a fire hazard. The recalled lights contain lithium-ion batteries that can overheat and ignite the plastic casing, which can lead to severe burn injuries. This recall comes after a reported death and another injury from smoke inhalation in 2023 when a light overheated and caused a house fire. The U.S. Consumer Product Safety Commission (CPSC) is aware of nine other incidents of battery overheating, with six resulting in fires and property damage.
The recalled lights were sold nationwide from October 2017 to January 2024 at hardware and home improvement stores as an alternative to wired fixtures in places like closets and cabinets. The model numbers to look for are RE1122, RE1145, RE1362, and RE1250, which can be found on a white sticker on the back of the light.
Medline Recalls 1.5 Million Adult Bed Rails After 2 Deaths Reported
In May 2024, Medline Industries issued a recall for 1.5 million portable adult bed rails after two entrapment deaths were reported. The Medline bed rail recall includes two models of the medical supply company’s “Bed Assist Bars” sold at major retailers like Walmart and Amazon between July 2009 and March 2024. Additional units were sold in Canada between February 2013 and March 2024, but no injuries have been reported.
The Medline bed rail recall involves the following two models:
- Medline Bed Assist Bar (Model #MDR107120)
- Medline Bed Assist Secure Bar (Model #MDR107125)
According to the U.S. Consumer Product Safety Commission (CPSC), users of the recalled Medline bed rails face serious entrapment hazards that can lead to injury or death. Individuals risk becoming entrapped within the bed rail itself or between the bed rail and the mattress.
Two reports of entrapment deaths have been reported in the U.S., including a 76-year-old woman who died in an Iowa senior nursing facility in 2019 and an 87-year-old woman who died at a South Carolina residential care facility in 2023.
Best Buy Insignia Pressure Cooker Recalled for Burn Hazard
In October 2023, Best Buy issued a recall for Insignia pressure cookers after reports of contents being expelled, causing injury. Consumers who have purchased this product should stop using it and contact Best Buy about a replacement. The recall includes:
Insignia Multi-Function Pressure Cookers
- Model numbers: NS-MC60SS8, NS-MC60SS9, or NS-MC80SS9
- Inner cooker pots, sold separately as replacements with model numbers NS-MCRP6NS9 and NS-MCRP6SS9
- Electric pressure cookers and inner cooker pots are six- and eight-quart capacities.

Thirty-one incidents had been reported at the time of the recall. Seventeen people suffered severe burn injuries.
Ten Babies Have Died Since Boppy Recalled Nursing Pillows and Sleepers in 2021
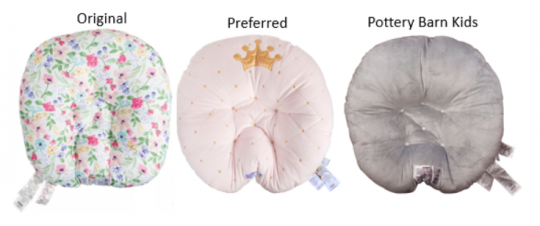
In 2020, the Consumer Products and Safety Commission (CPSC) issued a warning to parents and caregivers not to let babies fall asleep on Boppy nursing pillows and sleepers. After analyzing government data in 2021, Consumer Reports found seven more reports of infant deaths and one injury linked to the products, which led to Boppy issuing a recall in September 2021. Nearly two years after the recall, two more babies have died after falling asleep on the unsafe products. The CPSC made an announcement in June 2023 urging sellers to remove the products from secondhand marketplaces.
Target Recalls 4.9 Million Threshold Candles for Severe Burn and Laceration Risk

The recall involves Threshold-branded candles in glass jars that were sold in multiple sizes and scents. The recalled Threshold products were sold exclusively in Target stores and the Target website between August 2019 and March 2023. A complete list can be found on the Consumer Products and Safety Commission (CPSC) website.
According to the CPSC, Target has received 137 reports of the Threshold candles’ jars breaking or cracking during use. This included at least six lacerations and severe burn injuries.
Consumers who purchased the recalled Threshold candles should stop using them immediately. They can be be returned to any Target store for a full refund.
Vi-Jon, LLC Expands Recall of All Flavors of Magnesium Citrate Saline Laxative Oral Solution Due to Dangerous Microbe Contamination
This recall includes lemon, grape and cherry flavors and was designed to treat occasional constipation (bowel irregularity).
This product was sold nationwide is retail stores and wholesale outlets under multiple brand names, including:
- Best Choice
- Care One
- Cariba
- Cruz Blanc
- CVS
- Discount Drug Mart
- Equaline
- Equate
- Exchange Select
- Family Wellness
- Good Sense
- Harris Teeter
- HEB
- Health Mart
- Kroger
- Leader
- Major
- Meijer
- Premier Value
- Publix
- Quality Choice
- Rexall
- Rite Aid
- Signature Care
- Sunmark
- Swan
- Topcare
- Up & Up
- Walgreens
The recall was initiated after microbial testing identified the presence of Gluconacetobacter liquefaciens, which can cause serious, life threatening, invasive infections in immunocompromised individuals. As of July 25, 2023, the FDA is aware of at least three serious adverse events associated with this product.
BODYARMOR SportWater Recall for Plastic Pieces
BA Sports Nutrition LLC of Whitestone, NY has issued a recall for BODYARMOR SportWater drink because it may contain plastic pieces. The recall includes the following products with the “best by” date of 021624 and UNX L04:
BODYARMOR SportWater 24 in 700-milliliter bottles
BODYARMOR SportWater 12 in 1 Liter bottles;
BODYARMOR SportWater 24 in 20-ounce 6 packs
BODYARMOR SportWater 2×6 in 1 Liter bottles;
BODYARMOR SportWater 15 in 700 milliliter Club
BODYARMOR SportWater 15 in 1 Liter Club
Jarman’s Midwest Cleaning Systems Products Recalled for Methanol
Jarman’s Midwest Cleaning Systems Inc. of Canton, SD, has issued a recall for products contaminated with methanol. According to the recall, “substantial methanol exposure can result in nausea, vomiting, headache blurred vision, coma, seizures, permanent blindness, permanent damage to the central nervous system, or death.”

The recall includes:
- Alcohol Antiseptic 80% Topical Solution Hand Sanitizer Non-sterile Solution, all lots
- Isopropyl Alcohol Antiseptic 75% Topical Solution Hand Sanitizer Non-sterile Solution
The recalled products were sold in 1-gallon clear or white plastic bottles. Sometimes they were sold in boxes containing four, 1-gallon bottles.
Atovaquone Oral Suspension, USP Recall for Bacillus cereus contamination
Camber Pharmaceuticals, Inc. of Piscataway, NJ has issued a recall for Atovaquone Oral Suspension, USP 750mg/5mL due to potential Bacillus cereus contamination. In immune-compromised patients, Bacillus cereus can cause severe illnesses such as endocarditis and necrotizing soft tissue infections. At the time of the recall, the company stated it had not received any reports of illnesses.
The recalled product was sold nationwide to wholesalers, and retail and mail-order pharmacies. The recalled product has the following identifiers:
- NDC # is 31722-629-21
- UPC # 331722629218
- Lot # is E220182
- Expiration date of 12/2023
Paradise Grills Recalls Outdoor Kitchens After Burn Injury Reports
After two people suffered major burn injuries, Paradise Grills recalled about 18,000 models of their First Generation Outdoor Kitchens. Paradise Grills reported that liquid propane gas can accumulate inside the grill when the lid is closed, creating a fire and burn hazard. The grills were sold for $4000 to $15000 at showrooms and at home, boat, and RV shows nationwide from January 2009 to December 2020. Recalled models include the following:
- GX-3, GX-4, GX-5, GX-6, GX-7, GX-8, GX-9, GX-10, GX-11 GX12 and GX14
- Tahiti
- Fiji
- Tropicana
- Aruba 6 and Aruba 8
Death Prompts Warning for Baby Trend Sit N’ Stand Double and Ultra Strollers
After a baby died and another was injured, the U.S. Consumer Product Safety Commission (CPSC) issued a safety warning for Baby Trend Sit N’Stand Double and Ultra strollers with model numbers starting with “SS66” or “SS76.” The space in front of and behind the stroller’s pivoting front canopy can trap a child’s head or neck if another child climbs onto the stroller. Entrapment can also occur when the child is not properly restrained in the stroller’s front seat using all five points of the harness.
Baby Trend received one report of a 14-month-old child who died of asphyxiation when their neck became trapped between the front of the canopy tube and the armrest. In a separate incident, a 17-month-old child who was partially secured in the stroller suffered neck bruise injuries when they became trapped in the space between the back of the canopy tube and the back of the front seat.
The strollers were sold online at babytrend.com, amazon.com, and bedbathandbeyond.com in addition to retail stores including Walmart, Target, Kohls, and buybuy BABY. The CPSC recommends that caregivers remove and separately store the canopy when not in use, prevent children from playing on the strollers, and always fully secure children in the strollers with the built-in five-point harness.
EzriCare and Delsam Pharma Artificial Tears Recalled for Deadly Bacterial Contamination
EzriCare and Delsam Pharma brand artificial tear drops have been recalled after the CDC announced a link to VIM-GES-CRPA infections, a type of infection caused by antibiotic resistant Pseudomonas aeruginosa bacteria. The recalled EzriCare and Delsam products are manufactured in China and distributed through online and retail stores by Global Pharma Healthcare in the United States.
These infections are known to have injured at least 55 people so far, in some cases causing permanent blindness and at least one death.
Cases are known to have occurred in 12 states so far: 12 states: California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington, and Wisconsin.

Generac Generators Recalled for Finger Amputation and Crush Injury Risk
On July 29, 2021 the Consumer Products Safety Commision announced Generac was recalling all Generac® and DR® 6500 Watt and 8000 Watt portable generators. The recall was necessary because, due to a design defect, an unlocked handle could pinch consumers’ fingers against the generator frame when the generator was moved, posing finger amputation and crushing hazards.
Fisher-Price Recalls Rock ‘n Play Sleepers and Kids2 Recalls Rocking Sleepers After Additional Infant Deaths Reported
This recall includes all models of Fisher-Price Rock ‘n Play Sleepers and all models of Kids2 Rocking Sleepers. This is a re-announcement of recalls for the same models of rocking sleepers originally recalled in April, 2019.
Target Recalls Pillowfort Weighted Blankets
Target and the U.S. Consumer Product Safety Commission (CPSC) announced a recall for more than 200,000 Pillowfort Weighted Blankets due to the risk of suffocation.
Target received four reports of children getting trapped after unzipping and entering the blankets. Two children, a four-year-old girl, and a six-year-old girl died from asphyxia at Camp Lejeune in North Carolina.

Photo by CPSC
The recalled blankets were sold exclusively at Target retail stores and online at target.com. The item number can be found printed on a fabric tag on the blanket cover. Item numbers included in the recall include:
- 097-02-0140 (unicorn white)
- 097-02-0148 (space navy)
- 097-02-0361 (pink)
- 097-02-0363 (blue)
- 097-02-0364 (gray)
- 097-02-1603 (buffalo plaid red)
- 097-02-3904 (blue constellation)
- 097-02-3905 (unicorn pink)
The Laundress Recalls Laundry and Cleaning Products Due to Bacterial Contamination
The Laundress issued a recall for about 8 million units of laundry detergent and household cleaning products due to exposure to bacteria. According to the U.S. Consumer Product Safety Commission (CPSC), testing identified dangerous bacteria in the recalled products, including Burkholderia cepacia complex (B. cepacia), Klebsiella aerogenes, and multiple different species of Pseudomonas.
To date, the CPSC has received eleven reports of Pseudomonas infections. People can get sick when they inhale the bacteria or it enters the body through the eyes or a break in the skin. People with weakened immune systems, external medical devices, and underlying lung conditions are at high risk for infection.

Photo from The CPSC
The recalled products were sold between January 2021 and September 2022 online and at retailers including TheLaundress.com, Amazon.com, The Laundress, Bloomingdale’s, The Container Store, Saks Fifth Avenue, Target, Nordstrom, Jenni Kayne, Kith, Peruvian Connection, N.Peal, Brooklinen, and others.
All products have “The Laundress – New York” printed on the top of the label. Products that are part of the recall can be identified by the following lot codes.
- Lot codes that begin with the letter F and the last four digits are numbered 9354 or less
- Lot codes that begin with the letter H and the last four digits are numbered 2262 or less
- Lot codes that begin with the letter T and the last four digits are numbered 5264 or less

On March 31, 2023, The Laundress issued a recall for fabric conditioners. These products are contaminated with ethylene oxide, “a carcinogen that can cause adverse health effects if there is significant and direct long-term exposure.”
From 2011 through November 2022, the products were sold online at websites including TheLaundress.com and Amazon.com. They were also sold in stores at: The Laundress, Bloomingdale’s, The Container Store, Saks Fifth Avenue, Target, Nordstrom, Jenni Kayne, Kith, Peruvian Connection, N.Peal, Brooklinen, and other major retailers nationwide.
The Laundress is a subsidiary of Conopco Inc., d/b/a Unilever, of Englewood Cliffs, NJ.
Contact Our Attorneys
If you or a family member was injured by contaminated Laundress products, contact our bacterial injury legal team online or call us at 1-888-377-8900.
Ancheer E-bikes Recalled For Burn Injury Hazard

Photo from the CPSC
Ancheer recalled about 22,000 electric bikes due to the risk of a lithium-ion battery fire or explosion. There have been six reports of fires and explosions, including four with burn injuries. The recall involves e-bikes with the model number AM001907 found on the packaging and instruction manual. A cylindrical battery shaped like a water bottle distinguishes the model from other products. The recalled e-bikes were sold between January 2016 and June 2022 at the following retailers:
- aliexpress.com
- ancheer.shop
- amazon.com
- ebay.com
- newegg.com
- overstock.com
- rakuten.com
- sears.com
- walmart.com
- wish.com
EXACTECH Knee, Hip and Ankle Joint Implants Have Been Recalled
Hundreds of thousands of replacement joint implants, some in use since 2004, have been recalled by Exactech after it was discovered that defective packaging could allow oxygen to degrade the plastic in the joint prior to it being surgically implanted in patients. The affected joints can fail over time leading to serious injury and requiring their surgical removal and replacement with a new joint.
The recall includes OPTETRAK, TRULIANT and VANTAGE knee implants; Acumatch, NOVATION and MCS hip implants and components; and all Exactech VANTAGE ankle implants.
Additional information on the Exactech recalls can be found here
4moms Recalls MamaRoo Baby Swings and RockaRoo Rockers After Baby Dies

Photo from the CPSC
4moms recalled millions of MamaRoo baby swings and RockaRoo rockers after a 10-month-old baby died from asphyxiation. The CPSC recall says when the swings and rockers are not in use, their restraint straps can hang below the seat, causing crawling infants to become entangled in the straps.
There have been two reports of entanglement incidents with the MamaRoo swing, where one 10-month-old infant died from asphyxiation. Another 10-month-old infant suffered bruising to the neck before being rescued by a caregiver. No injuries associated with the RockaRoo rockers have been reported.
The 4moms recall includes the following baby swings and rockers, which were sold at BuyBuy Baby, Target, 4moms.com, and amazon.com from January 2010 to August 2022.
| Recalled MamaRoo Baby Swings | Recalled RockaRoo Baby Rockers |
|---|---|
| Versions 1.0 and 2.0 (model number 4M-005) | Model number 3M-012 |
| Version 3.0 (model number 1026) | |
| Version 4.0 (model number 1037) |
FDA Warns Caregivers Against Using Inflatable Neck Floats After Baby Dies, Another Hospitalized
The FDA issued a warning for caregivers not to use inflatable neck floats with babies for water therapy after one baby died and another was hospitalized.
Risks associated with using the neck floats include drowning, suffocation, and neck injuries. Babies with developmental delays or special needs such as spina bifida, SMA type 1, Down syndrome, and cerebral palsy have an increased risk of serious injury or death.
The neck floats are not FDA approved. Some companies have marketed their products for babies as young as two weeks old.
Caregivers have used neck floats during a baby’s bath, while a baby is swimming, and as a water therapy tool. The FDA has received reports of at least one death and one hospitalization associated with using neck floats.
The FDA’s recommendations for caregivers include the following:
- Do not use inflatable neck floats as a physical therapy tool for babies.
- Be aware that using neck floats as a floatation device for babies with special needs can lead to an increased risk of serious injury or death.
- Be aware that baby neck floats are not FDA approved.
- If a neck float has injured a baby in your care, report the incident to the FDA.
Medtronic Recalls HVAD Pump Implant Kit For Pump Weld Defect
Medtronic recalled 1,614 HVAD pump implant kits after a pump weld defect caused one death and two injuries. The pump implant kits are part of the HeartWare HVAD System, which is used in cardiac transplant patients to help the heart pump blood to the rest of the body. The recall includes the following models, distributed between October 11, 2006, and June 3, 2021:
- 1101
- 1103
- 1104
- 1104JP
- MCS1705PU
The pump weld defect was found after an inspection of explanted pumps that were returned to Medtronic. An analysis showed that moisture entered the center post of the pump, causing corrosion and internal magnets to demagnetize. Patients with the recalled devices may have symptoms that resemble pump thrombosis, including fatigue, dark urine, and yellowed eyes. The pump weld defect may lead to pump malfunction, major surgery for pump replacement, severe organ dysfunction, stroke, and death.
Bestar Recalls Wall Beds for Crush Injury Hazard, 1 Death Reported
Bestar recalled 129,000 wall beds due to serious impact and crush injury hazards after one death and 60 injuries were reported. In July 2018, a 79-year-old woman died from a spinal injury after a Bestar bed detached from the wall and fell on her. There have been 60 other reports of injuries from Bestar wall beds detaching and hitting consumers.
Bestar sold the wall beds online from June 2014 to March 2022 through wayfair.com, costco.com, cymax.com, and amazon.com. The recall includes the following queen and full-size models:
- Nebula
- PUR
- Versatile
- Edge
- Cielo
- Audrea
- Lumina
- Orion
- Novello
The U.S. Consumer Product Safety Commission (CPSC) recommends that consumers stop using the recalled wall beds immediately.
Better Homes & Gardens Aromatherapy Room Spray Sold at Walmart Linked to Melioidosis Illnesses and Deaths
The aromatherapy spray, which came in multiple scents, has been linked to at least four illnesses and two deaths. The spray was sold at Walmart stores in 18 states.
Tuberculosis Outbreak Linked to FiberCel Bone Repair (“Bone Graft”) Material
On June 2, 2021, after multiple patients contracted Tuberculosis, Aziyo Biologics recalled 154 units of its FiberCel Bone Matrix. The recalled product was distributed by Medtronic to hospitals in 20 states. Pritzker Hageman Tuberculosis infection disease lawyers are investigating and a Tuberculosis bone graft lawsuit is possible.
A second outbreak linked to Aziyo Biologics bone graft material, contaminated with tuberculosis was announced in July 2023. At least five people were sickened, one died and 36 people with grafts are being treated in case they received contaminated products.
Eco-Gel 200 and MediChoice Ultrasound Gel, Manufactured by Eco-Med, Recalled for Bacterial Contamination
The ultrasound gel has been recalled due to contamination by Burkholderia cepacia complex bacteria. At least 15 people have had infections linked to improperly sterilized Eco-Gel 200 and MediChoice ultrasound gel. More cases may still be identified. Exposure to this contaminated ultrasound gel may lead to blood infections, sepsis, and potentially death. If you or a loved one has been infected as a result of this contaminated ultrasound gel, contact our experienced medical product infection lawyers today.
Defective X-Stand ‘Silent Adrenaline’ and ‘Apache’ Treestands
X-Stand brand deer hunting tree stands sold between May 2017 through December 2018 have a cable assembly defect that can lead to corrosion and cable failure causing unexpected falls and serious injury. Multiple injuries have been reported and the product has been recalled.
Elmiron Lawsuit | Bladder drug can lead to serious eye damage and blindness
The drug Elmiron, sold by Janssen Pharmaceuticals for treating bladder pain has been shown to cause eye damage in up to 50% of patients taking it.
Meningitis from Food Poisoning and Contaminated Medical Products
Our law firm has settled several meningitis lawsuits against food and drug companies. We have (and are) representing clients with meningitis (brain infection) from Listeria monocytogenes and Salmonella, both bacteria that can contaminate food and cause severe illness. We have also settled cases for clients who got this kind of infection from contaminated medical products.
Mycobacteria Infection
People who contract nontuberculous Mycobacteria chimaera (NTM) may have a lawsuit. Our lawyers will need to gather evidence to determine if if there is a connection between the infection and a medical product or unsanitary conditions.
Endoscope Infection
Our lawyers are helping people who got an infection from an endoscope that was not cleaned adequately. There have been several outbreaks of hospital-acquired Carbapenem-resistant Enterobacteriaceae (CRE), involving Klebsiella species and Escherichia coli (E. coli).
Hospital Infection Outbreak
Our law firm helps clients with personal injury and wrongful death lawsuits against manufacturers and compounding pharmacies that sell contaminated medicine that causes outbreaks of illness from bacteria, viruses, fungi and parasites. Our cases against hospitals involve infection outbreaks.