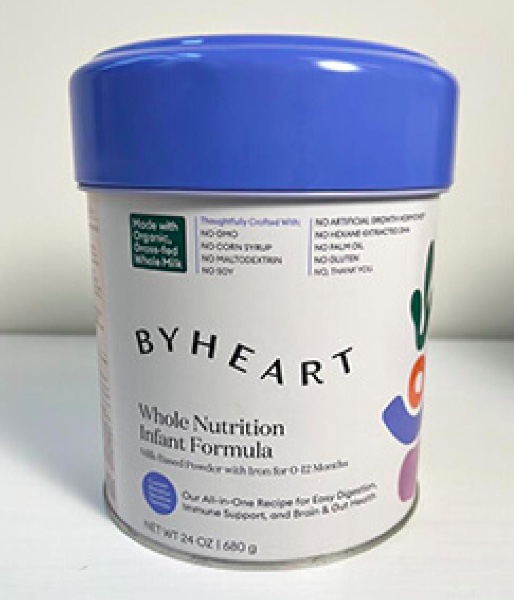
ByHeart has issued a recall for five batches of Whole Nutrition Infant Formula for potential Cronobacter cross-contamination risk. No formula that was distributed into commerce tested positive for Cronobacter and the company had received no reports of illness at the time of the recall.
The recall is not related to the company’s facility in Reading, PA where operations are continuing 24/7. According to the recall, ByHeart owns its entire manufacturing supply chain except final canning. The third-party packager that provides final canning found one sample collected from the facility tested positive for Cronobacter sakazakii. All of ByHeart’s infant formula packaged that day, and batches included in the first production on the following day were isolated for destruction and not distributed. However, the company decided to recall the entire production run out of an abundance of caution.
The recall includes:
- ByHeart Whole Nutrition Infant Formula, Milk-Based Powder with Iron for 0-12 Months
- 24 oz containers
- Batch numbers 22273 C1, 22276 C1, 22277 C1, 22278 C1, and 22280 C1
- “Use by” dated 01 JAN 24 or 01JUL 24
The recalled formula was distributed directly to customers.

Cronobacter bacteria can cause severe, life-threatening infections such as sepsis and meningitis. Symptoms of these infections include poor feeding, irritability, temperature changes, grunting breaths, abnormal movements, and yellowing of the skin or eyes. Seek immediate medical care if your infant displays these symptoms.
Contact the Pritzker Hageman Lawyers Today
Phone: 1-888-377-8900 | Text: 612-261-0856
The consultation is free and you never pay anything until we win for you.