Kratom (Mitragyna speciosa) has been linked to cases of Salmonella poisoning, and certain brands have been recalled.
Salmonella Lawsuit: 5 Reasons to Sue
Salmonella Outbreak Linked to Kratom
At least 87 people in 35 states have been sickened in this outbreak, according to the CDC. There are four strains of this bacteria involved in the outbreak:
- Salmonella I 4,[5],12:b:- (50 people sickened);
- Salmonella Javiana (5);
- Salmonella Okatie (16); and
- Salmonella Thompson (16).
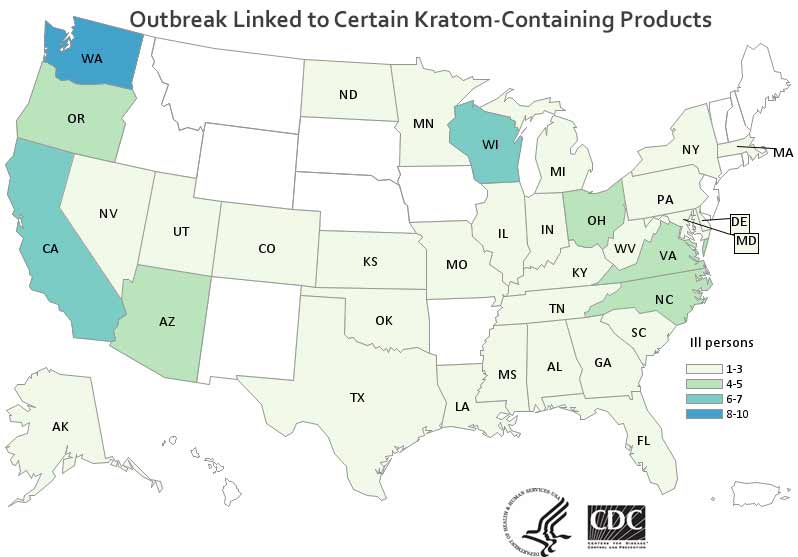
The outbreak has been going on for over a year. The first reported onset-of-illness date is January 21, 2017, and the most recently reported onset-of-illness date is February 24, 2018. It can take weeks for the CDC to report new cases, so the number of people sickened and the last reported onset-of-illness date may change.
Those sickened range in age from 6 to 67 years. Of 69 people with available information, 27 have been hospitalized.
I Want a Free Consultation with a Lawyer
Salmonella Found in Certain Kratom Products
State and local health officials have collected various leftover and unopened kratom products to test for Salmonella contamination.
In California health officials collected leftover Phytoextractum brand kratom powder from an ill person, and the outbreak strain of Salmonella I 4,[5],12:b:- was found in this sample. Prompted by these findings, PDX Aromatics recalled kratom powder sold online between January 18, 2018 and February 18, 2018. This recall is discussed below.
In Oregon and Utah investigators collected kratom powder from retail locations and online retailers where ill people reported purchasing kratom. Outbreak strains of Salmonella Okatie and Salmonella Thompson were found in these samples. No brand information was available for the kratom products collected in Oregon. The ill person in Utah purchased kratom powder from the website kratoma.com, according to the CDC.
Health officials in North Dakota and Utah collected leftover kratom powder from people who had been diagnosed with illnesses from Salmonella I 4,[5],12:b:-. The outbreak strain of Salmonella I 4,[5],12:b:- was identified in both samples of leftovers. The ill person in North Dakota purchased S.K. Herbalist brand kratom powder from the website soapkorner.com, and the ill person in Utah purchased kratom powder from the website kratoma.com, according to the CDC.
Kratom Product Recalls
Eclipse Kratom-Containing Powder Products
Tamarack Inc. of Roy, Utah recalled Eclipse Kratom-containing powder products. The recall was initiated after Tamarack Inc. was notified of positive Salmonella test results by the Food and Drug Administration. These products are not connected to the Salmonella outbreak, and no illnesses have been reported to date.
The affected powder products are packaged in plastic heat sealed pouches or plastic sealed bottles sold in one gram capsules and powder. Distribution of an estimated 120 units were sold directly to five retailers in Utah.
Tamarack has identified the supplier and source of contaminated product and has ceased the production and distribution of the product.
Kraken Kratom, Phytoextractum and Soul Speciosa
PDX Aromatics of Portland, Oregon (doing business as Kraken Kratom, Phytoextractum, and Soul Speciosa) also recalled certain kratom-containing powder products because of possible Salmonella contamination.
The first products recalled included powder products packaged in plastic heat sealed pouches and sold in 28 grams, 56 grams, and 112 grams. The products total an estimated 10,000 units and were sold directly to consumers via company websites between January 18, 2018 and February 18, 2018. The products description and lot codes are listed below.
| Brand | Product name | Net Wt |
|---|---|---|
| Kraken Kratom | Red Dragon Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Red Vein Borneo Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Red Vein Sumatra Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Red Vein Thai Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Super Indo Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Maeng Da Thai Kratom Powder (Horn Red) | 28 g, 56 g, 112 g |
| Phytoextractum | Red Dragon Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Red Vein Borneo Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Red Vein Sumatra Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Red Vein Thai Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Super Indo Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Maeng Da Thai Kratom Powder (Horn Red) | 28 g, 56 g, 112 g |
| Soul Speciosa | Red Dragon Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Red Vein Sumatra Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Red Vein Thai Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Super Indo Kratom Powder | 28 g, 56 g, 112 g |
| Recalled products have any of the following LOT codes (represent packaging dates): | ||||
|---|---|---|---|---|
| LOT 20180118 | LOT 20180125 | LOT 20180201 | LOT 20180207 | LOT 20180214 |
| LOT 20180119 | LOT 20180126 | LOT 20180201 | LOT 20180208 | LOT 20180215 |
| LOT 20180120 | LOT 20180127 | LOT 20180202 | LOT 20180209 | LOT 20180216 |
| LOT 20180121 | LOT 20180128 | LOT 20180203 | LOT 20180210 | LOT 20180217 |
| LOT 20180122 | LOT 20180129 | LOT 20180204 | LOT 20180211 | LOT 20180218 |
| LOT 20180123 | LOT 20180130 | LOT 20180205 | LOT 20180212 | |
| LOT 20180124 | LOT 20180131 | LOT 20180206 | LOT 20180213 | |
According to the press release:
“PDX Aromatics has identified a supplier in our supply chain as the source of Salmonella. The company has removed that supplier from our supply chain and all associated products from our facility. We have ceased distribution of products in order to perform a facility audit and have initiated a voluntary recall. Working in cooperation with the FDA, the company will destroy all recalled product upon return.”
An expanded recall involved certain kratom white vein powder and capsule products and red vein powder products shipped between 1/18/2018 and 3/8/2018. The list is below:
WHITE VEIN powder and capsule EXPANDED recalled products:
| Brand | Product name | Net Wt |
|---|---|---|
| Kraken Kratom | Maeng Da Thai Kratom Powder (White Vein) | 28 g, 56 g, 112 g |
| Kraken Kratom | White Vein Borneo Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | White Vein Sumatra | 28 g, 56 g, 112 g |
| Kraken Kratom | White Vein Borneo Kratom Capsules | 28 g, 56 g, 112 g |
| Phytoextractum | White Maeng Da Powder | 28 g, 56 g, 112 g, 225g |
| Phytoextractum | White Borneo Powder | 28 g, 56 g, 112 g, 225 g |
| Phytoextractum | White Vein Sumatra | 28 g, 56 g, 112 g |
| Phytoextractum | White Maeng Da Thai Capsules | 28 g, 56 g, 112 g |
| Soul Speciosa | Borneo Kratom Powder (White Vein) | 28 g, 56 g, 112 g |
| Soul Speciosa | Sumatra Kratom Powder (White Vein) | 28 g, 56 g, 112 g |
| Soul Speciosa | White Vein Borneo Kratom Capsules | 28 g, 56 g, 112 g |
WHITE VEIN powder and capsule EXPANDED recalled products have any of the following LOT codes:
| LOT 20180216 | LOT 20180221 | LOT 20180226 | LOT 20180303 | LOT 20180308 |
| LOT 20180217 | LOT 20180222 | LOT 20180227 | LOT 20180304 | |
| LOT 20180218 | LOT 20180223 | LOT 20180228 | LOT 20180305 | |
| LOT 20180219 | LOT 20180224 | LOT 20180301 | LOT 20180306 | |
| LOT 20180220 | LOT 20180225 | LOT 20180302 | LOT 20180307 |
RED VEIN powder EXPANDED recalled products:
| Brand | Product name | Net Wt |
|---|---|---|
| Kraken Kratom | Red Dragon Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Red Vein Borneo Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Red Vein Sumatra Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Red Vein Thai Kratom Powder | 28 g, 56 g, 112 g |
| Kraken Kratom | Super Indo Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Maeng Da Thai Kratom Powder (Horn Red) | 28 g, 56 g, 112 g |
| Phytoextractum | Red Dragon Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Red Vein Borneo Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Red Vein Sumatra Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Red Vein Thai Kratom Powder | 28 g, 56 g, 112 g |
| Phytoextractum | Super Indo Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Maeng Da Thai Kratom Powder (Horn Red) | 28 g, 56 g, 112 g |
| Soul Speciosa | Red Dragon Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Red Vein Sumatra Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Red Vein Thai Kratom Powder | 28 g, 56 g, 112 g |
| Soul Speciosa | Super Indo Kratom Powder | 28 g, 56 g, 112 g |
RED VEIN powder EXPANDED recalled products have any of the following LOT codes:
| LOT 20180219 | LOT 20180228 |
| LOT 20180220 | LOT 20180301 |
| LOT 20180221 | LOT 20180302 |
| LOT 20180222 | LOT 20180303 |
| LOT 20180223 | LOT 20180304 |
| LOT 20180224 | LOT 20180305 |
| LOT 20180225 | LOT 20180306 |
| LOT 20180226 | LOT 20180307 |
| LOT 20180227 | LOT 20180308 |
The company issued a second expanded recall of certain red vein and green vein kratom powder and capsule products after additional positive findings of Salmonella associated with PDX Aromatics’ products following the FDA’s investigation. To date the company is aware of four confirmed cases of Salmonella associated with consumption of red and white vein products.
The expanded recall includes certain red vein powder and capsule products shipped between 12/1/2017 and 3/8/2018 and certain green vein powder and capsule products shipped between 9/18/2017 and 11/1/2017 and between 2/9/2018 and 3/8/2018. Recalled products were sold to two customers in the United Kingdom and to one customer in each of the following countries: Ireland, Japan, Norway, and Turkey.