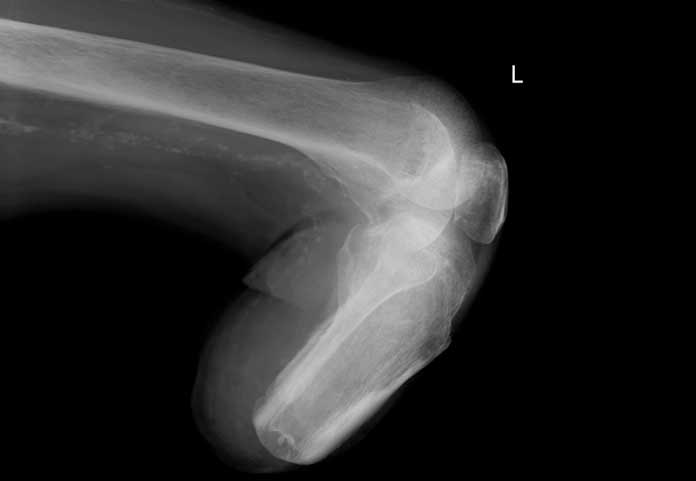
Based on new data from two large clinical trials, the FDA “concluded that the type 2 diabetes medicine canagliflozin (Invokana, Invokamet, Invokamet XR) causes an increased risk of leg and foot amputations” (FDA Drug Safety Communication). The FDA is now requiring a new Boxed Warning describing this risk to be added to the drug labels of these diabetes medications. This is not a recall, but it provides doctors and patients information on the amputation risk.
2 Large Clinical Trials Find Two-Fold Increased Risk of Lower Limb Amputations
Two clinical trials found an approximate 2-fold increased risk of lower limb amputations associated with a medicine called canagliflozin, sold by Janssen Pharmaceuticals under the brand names Invokana® and Invokamet®, both registered trade names.
These were large, randomized, placebo-controlled trials evaluating patients with type 2 diabetes who had either established heart disease or were at risk for it. These clinical trials were named CANVAS (Canagliflozin Cardiovascular Assessment Study) and CANVAS-R (A Study of the Effects of Canagliflozin on Renal Endpoints in Adult Participants With Type 2 Diabetes Mellitus).
The combined amputation data is as follows:
- amputations of the toe and mid-foot (99 out of 140 patients with amputations receiving canagliflozin in the two trials);
- amputations involving the leg, below and above the knee (41 out of 140 patients with amputations receiving canagliflozin in the two trials).
Some patients had more than one amputation, including a few involving more than one limb.
The most common medical events leading to amputation included lower limb infections, gangrene, diabetic foot ulcers, and ischemia. The risk of amputation was highest in patients with a baseline history of prior amputation, peripheral vascular disease, and neuropathy.
The sponsor of both studies was Janssen Research & Development, LLC.
CANVAS Findings
“In CANVAS, the risk of lower limb amputations was 5.9 amputations per 1,000 patients per year for canagliflozin compared to 2.8 amputations per 1,000 patients per year for placebo” (FDA).
This study was started in December of 2009 and the primary completion date was February 22, 2017.
CANVAS Amputations
The CANVAS trial showed that over a year’s time, the risk of amputation for patients in the trial were equivalent to:
- 5.9 out of every 1,000 patients treated with canagliflozin (sold under the brand names Invokana and Invokamet);
- 2.8 out of every 1,000 patients treated with placebo.
CANVAS-R Findings
“In CANVAS-R, the risk of lower limb amputations was 7.5 amputations per 1,000 patients per year for canagliflozin compared to 4.2 amputations per 1,000 patients per year for placebo. The risk of lower limb amputations was observed at both the 100 mg and 300 mg doses” (FDA).
This study was started in January of 2014 and the primary completion date was February 21, 2017.
The CANVAS-R trial showed that over a year’s time, the risk of amputation for patients in the trial were equivalent to:
- 7.5 out of every 1,000 patients treated with canagliflozin
- 4.2 out of every 1,000 patients treated with placebo.
What is Canagliflozin, the Active Ingredient in Invokana and Invokamet?
The following information is from the U.S. Food and Drug Administration:
- Canagliflozin is a prescription medicine that is used with diet and exercise to lower blood sugar in adults with type 2 diabetes. It belongs to a class of drugs called sodium-glucose cotransporter-2 (SGLT2) inhibitors.
- Canagliflozin is available as a single-ingredient product under the brand name Invokana, and also in combination with the diabetes medicine metformin under the brand names Invokamet and Invokamet XR.
- This drug lowers blood sugar by causing the kidneys to remove sugar from the body through the urine.
- Other side effects, in addition to the increased risk of medical events requiring amputation, include low blood pressure, a condition of too much acid in the blood called ketoacidosis; kidney problems; a high amount of potassium in the blood; serious urinary tract infections; low blood sugar when combined with other prescription diabetes medicines; yeast infections; bone breaks; and increased cholesterol.
More research is needed regarding the safety of these type 2 diabetes medications. We will alert our readers if there is a recall.
Several lawsuits against Janssen for alleged injuries from Invokana have been filed in the United States, although none of the injuries were amputations. “These involve allegations that ingestion of the drug Invokana may cause a variety of injuries, including diabetic ketoacidosis and kidney damage, and that defendant Janssen Pharmaceuticals, Inc., which developed and manufactured the drug, failed to adequately test the drug and warn of its risks” (MDL-2750 Transfer Order). These suits, which are not a class action, have been consolidated for pretrial procedures only in a process called multidistrict litigation (MDL). Our law firm did not file these lawsuits.