The FDA is reporting that the ongoing investigation of New England Compounding Center (NECC) has identified a patient with 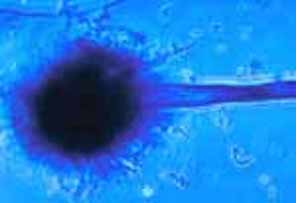
In addition, two transplant patients with Aspergillus fumigatus infection who were administered NECC cardioplegic solution during transplant surgery have been reported. Investigation of these patients is ongoing; and there may be other explanations for their Aspergillus infection. Cardioplegic solution is used to induce cardiac muscle paralysis during open heart surgery to prevent injury to the heart.
FDA advises healthcare professionals to follow-up with patients who were administered any injectable medication from or produced by NECC, including injectable ophthalmic drugs used in conjunction with eye surgery, or a cardioplegic solution purchased from or produced by NECC after May 21, 2012. Healthcare professionals and medical care organizations should inform patients who received the NECC products noted above of the symptoms of possible infection and instruct patients to contact their healthcare provider immediately if they experience any of these symptoms.