When a massive Listeria recall triggered an FDA inspection at a Maryland ice cream plant, it set off a series of events that culminated in U.S. District Court this week. On March 3, the U.S. Food and Drug Administration (FDA) filed a complaint of permanent injunction seeking to bar the owners, officers and employees of Totally Cool Inc. from restarting operations at the Owings Mills facility linked to the Listeria recall, or at any new location, until FDA inspectors confirm that conditions comply with food safety laws.
How Did a “Resident Strain” of Listeria Take Hold at Totally Cool?
The FDA says the Owings Mills facility has a “resident strain” of Listeria monocytogenes, meaning the same strain of bacteria was isolated from multiple samples over time. In 2024, the agency used whole genome sequencing, which can determine the genetic “fingerprint” of bacteria, on isolates collected during various inspections. The results showed that the same strain was present in samples collected from the plant during inspections in 2017, 2019, and 2024. The strain also turned up in an isolate from a person who developed listeriosis in 2015.
A variety of Listeria species are harmless to humans, but their presence can indicate that conditions are favorable for Listeria monocytogenes, which is deadly. Nearly everyone with a Listeria monocytogenes infection requires hospitalization, and about 20 percent of cases are fatal. Among pregnant women, it can cause miscarriage or stillbirth, even if the expectant mother experiences only flu-like symptoms.
Unlike other bacteria, Listeria thrives in cold environments. It can colonize on damp surfaces such as floors, floor drains, and processing equipment, and in condensation, standing water, and food residues.
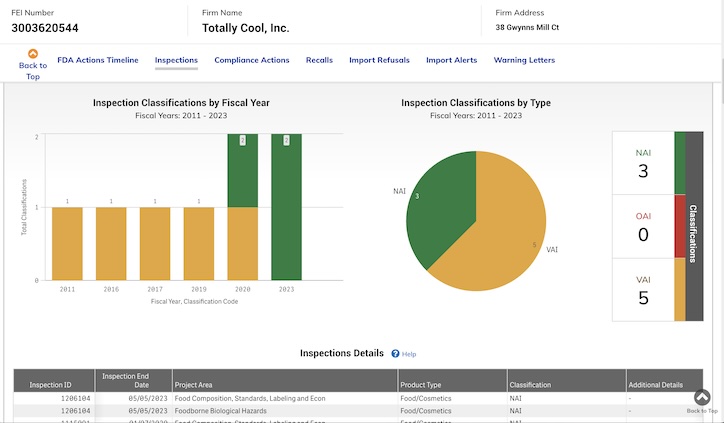
8 FDA Inspections
Between 2011 and 2024, the FDA inspected the Owings Mills facility eight times. Some information about the first seven inspections is available on the FDA website and some was cited in the complaint.
At each visit, a violation for foodborne biological hazards was cited, this includes “all field activities directed towards reducing the incidence of microbiological hazards, filth, decomposition and foreign objects in the nation’s food supply.”
During these visits, inspectors found Listeria and/or conditions that facilitate its growth such as:
- Facility was not in good repair
- Plumbing that constituted a source of contamination to water supplies.
- A cracked floor
- Inadequate environmental sampling and monitoring because locations and frequency were not identified
- No evidence that corrective actions were taken after Listeria was found
- Poor handwashing among employees
- Dripping condensate
- Pools of standing water and debris on the floor
- Excessive product buildup on equipment
Despite these findings, none of the violations issued were “official,” which would have been indicated in red on the charts below. Most were voluntary actions, and in three instances, no action was indicated.
.
But, in 2024, when the FDA again found Listeria at the plant, it requested a recall for all products made there. This included ice cream and sorbet sold in pints, cones, sandwiches, and cakes under 13 brand names – AMA Fruits, Abilyn’s, ChipWich, Cumberland Farms, Dolcezza, Friendly’s, the Frozen Farmer, Hershey’s, Jeni’s, La Salle, Marco, Tahraka, and Yelloh.

When the recall was issued on June 25, 2024, Totally Cool halted production at the plant. On July 8, 2024, the FDA suspended the company’s food facility registration. On August 23, 2024, the company filed for bankruptcy.
Since the suspension of the registration, the FDA has exchanged correspondence with the company about proposed corrective actions, but they haven’t been sufficient, the FDA says in the complaint.