Contact our Medical Product Liability Attorneys
Fluid leaks causing unexpected pump shutdown or the inability to initiate therapy have prompted the recall of Cardiosave Hybrid and Cardiosave Rescue Intra-Aortic Balloon Pumps (IABPs). At the time the recall was issued, the U.S. Food and Drug Administration (FDA) had received 71 complaints. One death was reported.
The recall includes:
-
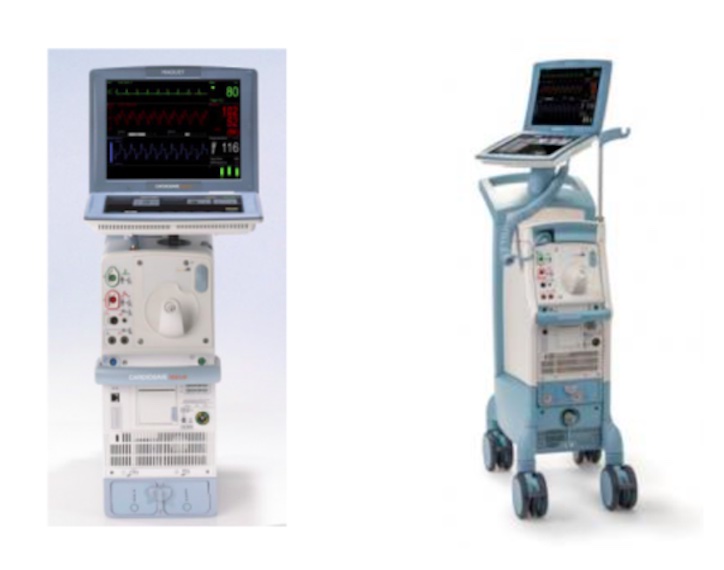
- Datascope/Getinge/Maquet Cardiosave Hybrid
- Cardiosave Rescue Intra-Aortic Balloon Pump (IABP)
These recalled products were manufactured between December 2011 to the present and distributed in the U.S. from March 6, 2012, to October 21, 2021.
Contact the Pritzker Hageman Medical Product Legal Team
Phone: 888-377-8900 | Text: 612-261-0856
Contact our Medical Product Liability Attorneys
The consultation is 100% free and you never pay us anything ever unless we collect money damages for you
FDA Recommendations
Cardiosave Hybrid and Rescue IABPs are used with patients undergoing cardiac and non-cardiac surgery, and to treat adult patients with acute coronary syndrome or complications of heart failure.
Getinge/Datascope/Maquet plans to plans to correct all 4,338 Cardiosave IABP devices in the field. These modifications which will include various internal and external component upgrades will be installed by a company service representative.
On November 15, 2021, Getinge/Datascope/Maquet notified its customers of the problem in an “Urgent Medical Device Correction Letter.” The letter instructed customers to:
- Determine if they had any Cardiosave Hybrid or Rescue IABPs.
- Follow the “Instructions For Use.”
- Never place fluids on top of the unit.
- Immediately wipe clean any spills.
- Use the Plastic Weather Display and Rescue Cover any time the Cardiosave Rescue IABP is used outdoors, especially when there is the possibility of wet weather.
Medical Product Liability Lawyers
We help people who are critically injured by unsafe medical products get the justice and full compensation they deserve. Our law firm has been representing people injured by defective medical products for the past 40 years. Pritzker Hageman was awarded a “Best Lawyers in America” award in mass tort litigation for our work representing clients with failed hip and knee implants.
If you would like to talk to a lawyer about your case, please call 1-888-377-8900, text 612-261-0856, or fill out the form below. Consultations are free and there is no obligation. A recall is not necessary to file a lawsuit and win a settlement.